The alkali metals are some of the most reactive elements in the periodic table. The alkali metals include: lithium, sodium, potassium, rubidium, cesium, and francium. Although often listed in Group 1 due to its electronic configuration, hydrogen is not technically an alkali metal since it rarely exhibits similar behavior. The word "alkali" comes form the Arabic word "al qali," meaning "from ashes". Similarly he word potassium is derived from potash. (The name derives from pot ash, which refers to plant ashes soaked in water in a pot, the primary means of manufacturing the product before the industrial era.) The alkali metals react readily with water to form hydroxide ions, creating alkaline solutions (pH>7).
The Group 1 alkali metals are the first column of the table. This means they are built upon their previous element, a noble gas. The noble gases have branches that are capped with neutral endings. Therefore they cannot react. The next step to build on a noble gas is to add two protons to one of the neutral endings and convert it into a lithium nuclet. This gives each of the alkali metals a valence of 1 and makes them highly reactive.
Lithium
Lithium follows the noble gas helium. It's most abundant isotope is lithium 7 which means it's nucleus has 7 protons. Lithium is the first solid element. It has one ring of five which gives it a valence of 1.
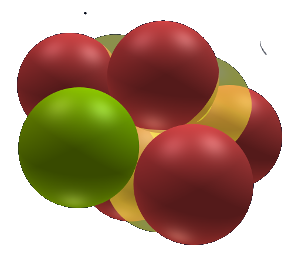
Sodium
Sodium follows the noble gas neon. It consists of one backbone nuclet which has a neutral ending on one side (green) and a lithium nuclet (red) on the other side. The neutral ending cannot react and does not effect valence. The lithium nuclet has a valence of 1 due to its one ring. This results in sodium having a valence of one.
Potassium
Potassium is the next element after Argon. It has two backbone nuclets. There are 3 exposed endings. Two of the endings are neutral endings (green) and one is a lithium nuclet (red) with a valence of 1. This results in Potassium having a valence of 1.
- Log in to post comments