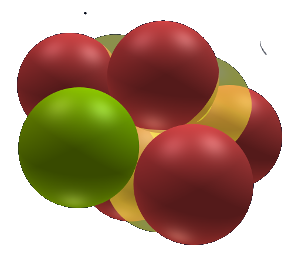
Hydrogen 3
Atom -
3 years 10 months ago
Hydrogen 3 or Tritium (T) as it is also known is an unstable isotope with 3 protons in a row and 2 inner electrons that have taken their place between 2 protons.
T will decay to He3
| Element | |
| Valence | *1, 0, -1 |
| Stability |
|
| Isotope | |
| Abundance | |
| Half Life |
12.32 Y
|
| Decay | |
| Protons |
3
|
| Inner Electrons |
2
|
| Outer Electrons |
1
|
| Nuclear Spin |
1/2+
|
| Mass Actual |
3.0160 AMU
|
| Mass H Norm |
2.9926 AMU
|
| Mass Calc |
3.0235 AMU
|
| BE Nucleon |
2827.26 KeV
|
| BE Actual |
8.48 MeV
|
| SAM Lines |
4.00
|
| BE SAM Lines |
8.90 MeV
|
| BE Difference |
95.30%
|
| AN-ISOTOPE |
1: 3
|
| Nid | 314 |
Atomic structure
N0:
state: hydrogen
protons:
P0:
P1:
P2:
electrons:
E01: {protons: [P1, P0]}
E02: {protons: [P2, P1]}
state: hydrogen
protons:
P0:
P1:
P2:
electrons:
E01: {protons: [P1, P0]}
E02: {protons: [P2, P1]}