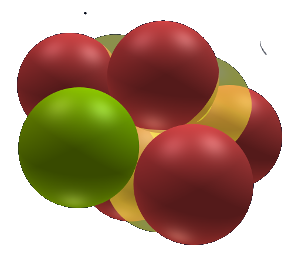
Fluorine 19
Fluorine 19 is the only stable isotope. The reason Fluorine is 19 and not 18 can be seen in the structure here. One side of the Carbon backbone nuclet has for the first time a so called "neutral 5-ending". The ending is capped by two Deuterium nuclets and has a fifth extra neutron that support that side from collapsing or re-balancing to the other side of the Carbon. This particular ending can be seen as the extra needed structural integrity to keep the shape stable.
Note that we see here that the both sides need to be in balance.
IF Fluorine were to have a full Lithium nuclet on one side (same as with Oxygen 18) then F 19 would not have the properties it has. namely -1.
A Li nuclet would have the +1 value and the remaining exposed Carbon grow point would have +1/+2 as valence value. This does not resemble Fluorine.
It seems the 2 D nuclets on one side need to be stabilized with the fifth extro neutron before the other side can be occupied. Before a true nuclet can be formed the other side needs to have a full neutral 5-ending.
| Element | |
| Valence | 0, *-1 |
| Stability |
|
| Isotope | |
| Abundance |
100.00 %
|
| Half Life |
Stable
|
| Decay | |
| Protons |
19
|
| Inner Electrons |
10
|
| Outer Electrons |
9
|
| Nuclear Spin |
1/2+
|
| Mass Actual |
18.9984 AMU
|
| Mass H Norm |
18.8509 AMU
|
| Mass Calc |
19.1487 AMU
|
| BE Nucleon |
7779.02 KeV
|
| BE Actual |
147.80 MeV
|
| SAM Lines |
69.00
|
| BE SAM Lines |
153.53 MeV
|
| BE Difference |
96.27%
|
| AN-ISOTOPE |
9: 19
|
| Nid | 255 |
state: initial
protons:
P0:
P1:
P2:
P3:
P4:
P5:
P6:
P7:
P8:
P9:
P10:
P11:
P12:
P13:
P14:
P15:
P18:
P19:
neutrons: [U00]
electrons:
E01: {protons: [P18, P19]}
E02: {protons: [P9, P0]}
E03: {protons: [P8, P5]}
E04: {protons: [P4, P1]}
E05: {protons: [P6, P2]}
E06: {protons: [P3, P10]}
E07: {protons: [P11, P7]}
E08: {protons: [P15, P14]}
E09: {protons: [P13, P12]}